Day :
- Medicinal Chemistry|Pharmaceutical Sciences

Chair
Matteo Micucci
Alma Mater Studiorum University of Bologna, Italy

Co-Chair
Shimon Ben-Shabat
Ben-Gurion University of the Negev, Israel
Session Introduction
Malkhaz Getia
Tbilisi State Medical University, Georgia
Title: Quantification of some medical preparations of the plant origin
Time : 11:10-11:35

Biography:
Malkhaz Getia has completed his PhD from Tbilisi State Medical University and Post-doctoral studies from University of Quebec at Chicoutimi, University of Liege and University of Marseille. He is a Scientific Researcher at the TSMU I K Institute of Pharmacochemistry. He has published more than 22 papers in reputed
journals and has been serving as an Editorial Board Member of reputed journal.
Abstract:
Among the biologically active compounds there are various triterpene glycosides (saponins). In recent years some crude extracts from various species of Georgian flora were obtained at the TSMU I Kutateladze Institute of Pharmacochemistry (Tbilisi, Georgia), namely from the leaves of Colchis ivy (Hedera colchica (K. Koch) – extract with antiulcer activity, from the leaves of Fatsia japonica extract with anti-rheumatic activity (Fatsiflogin) and from the leaves of Hedera helix seu H. caucasigena extract with bronchospasmolytic properties (Causuron). Hederacolchiside F (HcF), Fatsiosid D and Hederasaponin C (HsC) were chosen as the biological and chemical markers for the biological active substances. The UV detection is performed at 205 nm. The chromatographic separation was achieved using a reversed phase column C-18. NMR: Structure elucidation of the markers were carried out using 1H NMR (Bruker Avance 400MHz), 13C (Bruker Avance 100 MHz). The proposed HPLC methods are linear in the range studied (r2>0.999) for all the analyses. Precision, sensitivity and linearity are satisfactory in the range studied. Finally, new, simple, sensitive and reproducible HPLC methods have been developed and validated for the simultaneous quantification of HcF, Fatsiosid D and HsC in the crude extract of Colchis ivy, Fatsiflogin and Causuron.
Shimon Ben-Shabat
Ben-Gurion University of the Negev, Israel
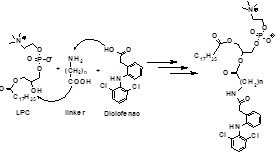
Title: Phospholipid-based prodrugs for the treatment of IBD: Drug targeting strategy
Time : 11:35-12:00

Biography:
Shimon Ben-Shabat has his expertise in Bio-organic and Medicinal Chemistry combining the following areas: Design and synthesis (structure-activity relationship), drug delivery approaches (pro-drugs), targeting and mechanistic studies and bio-analytical studies. His work centers on the relationships between chemistry and biological activity, including evaluation on different disease models
Abstract:
Phospholipase A2 (PLA2) expression/activity is significantly elevated in inflamed intestinal tissue in inflammatory bowel disease (IBD), Crohn’s disease and ulcerative colitis. PLA2 hydrolyses the sn-2 fatty acyl bond of phospholipids (PL) liberating a fattyacid and a lysophospholipid. By replacing the sn-2 positioned fatty-acid with a drug, PLA2 may be exploited as a prodrug activating enzyme, liberating the free drug from the PL-complex. Therefore, orally delivered PL-based prodrugs will release the free drug at the inflamed sites, effectively targeting the regions of intestinal inflammation. We have utilized a modern computational approach to simulate the PLA2-mediated activation using the candidate drug, and to predict the most appropriate linker length. We have synthesized PL-diclofenac conjugates and shown in-vitro activation of these synthesized conjugates by isolated bee venom PLA2 and conditioned medium from inflamed Caco-2 cell line. We showed that depending on the linker length between the PL and diclofenac, PLA2 could be exploited as the activating enzyme in-vitro, liberating the free diclofenac from the PL complex. We have compared the computational calculations to our experimental data, and obtained excellent correlation between the in-silico predictions and the in-vitro experiments. The proposed research may significantly improve drug therapy in IBD patients, enabling higher efficacy and lower toxicity profiles.
Recent Publications
1. A Dahan, S Ben-Shabat, N Cohen, S Keinan, I Kurnikov, A Aponick, E M Zimmermann (2016) Phospholipid-based prodrugs for drug targeting in inflammatory bowel disease: Computational optimization and in-vitro correlation. Curr. Top. Med. Chem. 16: 2543-8.
2. A Dahan, E Zimmermann, S Ben-Shabat (2014) Modern prodrug design for targeted oral drug delivery. Molecules 19: 16489-16505.
3. Wolk, S Epstein, V Ioffe-Dahan, S Ben-Shabat, A Dahan (2013) New targeting strategies in drug therapy of inflammatory bowel disease: Mechanistic approaches and opportunities, Expert Opin. Drug Deliv. 10: 1275-1286.
4. A Dahan, G L Amidon, E M Zimmermann (2010) Drug targeting strategies for the treatment of inflammatory bowel disease: A mechanistic update. Expert Rev. Clin. Immunol. 6: 543-550.
5. A Dahan, R Duvdevani, E Dvir, A Elmann, A Hoffman (2007) A novel mechanism for oral controlled release of drugs by continuous degradation of a phospholipid prodrug along the intestine: In-vivo and in-vitro evaluation of an indomethacin-lecithin conjugate. J. Control Release 119: 86-93.
Zhongli Gao
Sanofi, USA
Title: Drug design via a scaffold hopping strategy: Case studies on discovery of clinical candidates H3 receptor antagonist and β-tryptase inhibitor
Time : 12:00-12:25

Biography:
Zhongli Gao is currently a Senior Principal Scientist at Sanofi. He received his PhD in Organic Chemistry from the City University of New York in 1993. Upon graduation, he carried out his Post-doctoral Research on the total synthesis of spinosyn-A supervised by Professor Paquette at Ohio State University. He joined Hoechst Pharmaceuticals, one of the predecessor companies of Sanofi in 1995. He has worked on a broad range of disease areas in CNS, respiratory, inflammation, oncology, and rare disease involving GPCRs, proteases, enzymes, kinases, ion channels and transporters. He has led many projects in advancing compounds into clinical and preclinical decisions.
Abstract:
Scaffold hopping is a technique that generates compounds containing a topologically different scaffold from the parent compound, but with similar or improved activity and other properties. This can be done intellectually by medicinal chemists or by computational algorithm. The technique is based on topological pharmacophore models developed from SAR of the current lead. In scaffold hopping, medicinal chemist’s insights into pharmacophore and SAR are crucial in this iterative process. Scaffold hopping can be used to generate new chemical entity; overcome patent or other limitations for the current leads; generate differentiated series of chemical matters. Common approaches include heterocyclic replacements, bonds formation and cleavage, and topology-based hopping. This presentation will describe medicinal chemistry of H3 receptor antagonist and tryptase inhibitor programs via using scaffold hopping strategy to generate multi-genesis of chemical leads. Through optimization of the leads, clinical candidates were identified. The profile of the candidates and in vivo effects in disease animal models will also be briefly discussed.
Matteo Micucci
Alma Mater Studiorum University of Bologna, Italy
Title: Nutraceuticals in gastrointestinal ailments: An emerging paradigm
Time : 12:25-12:50

Biography:
Matteo Micucci has completed his PhD from Bologna University. He is working on Medicinal Chemistry and Nutraceuticals in the Department of Pharmacy and Biotechnology, University of Bologna. He was a Guest Scientist at University of Naples Federico II, in the research group coordinated by Professor Ernesto Fattorusso. He has published 25 papers in reputed journals.
Abstract:
Inflammatory bowel disease (IBD) is a chronic gastrointestinal disorder, of multifactorial origin. The pathogenetic mechanisms consist of immune dysregulation, altered intestinal microflora, oxidative stress, defects in the gastrointestinal mucosal barrier and increased permeability, altered intestinal motility, whose interplay leads to the onset of a state of chronic mucosal inflammation. The drugs for IBD treatment include corticosteroids, immunosuppressants, and anti-tumor necrosis factor (TNF)-α antibodies, and new therapeutic molecules, that increase the risk of opportunistic infections and malignancies. Furthermore, their efficacy decreases over time and highlights the need to identify new molecular targets for IBD therapy. Scientific research aims at identifying tools able to affect several targets, with minimal side effects. Nutraceutical identifies foods, or food phytochemicals, of animal or vegetal origin, with pharmaceutical properties. Many vegetal extracts determine several effects towards the gastrointestinal tract, which may result in clinical benefits in subjects suffering from IBD. Castanea sativa Mill. bark extract (ENC), containing high amounts of hydrolyzable tannins, inhibits spasmodic contractions, induced by carbachol, histamine, potassium chloride, and barium chloride in guinea pig ileum and by carbachol or serotonin in guinea pig proximal colon. Furthermore, ENC increases gallbladder contraction and relaxes the sphincter of Oddi, suggesting its chronic administration may result not only in a restoration of gastrointestinal contractility, but also in the prevention of gallstone disease. Also Acacia catechu Willd. extract (ACE) was investigated. ACE contains high amounts of catechins, such as (-)-Epicatechin and (+)-Catechin. This extract decreases, in a concentration-dependent manner, colon and ileum spontaneous contractility. In addition, ACE exerts a calcium antagonistic effect, more potent in proximal colon than in ileum. Furthermore, it exhibits antimicrobial effects against Campylobacter jejuni, Escherichia coli, and Salmonella spp., without inhibiting Bifidobacterium and Lactobacillus. These data support the use of ENC and ACE as co-adjuvant in the treatment of IBD.
Gaia Cecchia
Nutrition & Prevention, Italy
Title: Pathogenesis of alimentary diabetes: recovery by loss of 20% body weight, and by attainment of initial hunger as well as of low BG before meals
Time : 13:50-14:15

Biography:
Gaia Cecchia has done a course in Mathematics from the University of Florence. She graduated in Pharmaceutical Sciences in the year 2012 with a thesis in neuropharmacology with the title: “Memantine and topiramate in association with hypothermia as neuroprotective agents in the neonatal ischemic encephalopathy”.Since 2012, she is working at the ONLUS-No-profit association ‘Nutrition & Prevention’ with Professor Mario Ciampolini.
Abstract:
Background & Objective: We attempted to train two diabetic adults as we suggested in the first abstract. Diabetic people are different from healthy people in this: they don’t develop any hunger sensation after meal suspension.
Methods: Training: recognizing Hinitial Hunger and associate this sensation to low Blood Glucose (76.6 ± 3.7 mg/dL). We tried to implement this training in two obese, diabetic adults.The two subjects consumed meals devoid of fats and carbohydrates (Very Low Energy Diet, VLED) for 6 to 12 months.
Results: At recruitment the two diabetic subjects (out of two) showed a BMI of 39 and 33 and they neither developed a BG decline to 76.6 ± 3.7 mg/dL nor any hunger sensation after 2-days eating suspension. Then Both subjects lost 13%-20% of their initial body weight; they recovered 76.6 ± 3.7 mg/dL of BG and hunger sensations before one – three meals a day, i.e.: they went out of diabetes.
Conclusion: Diabetes develops by inveterate conditioned intake (when previous energy intake has not been fully exhausted before meals), excessive fattening, excessive post-absorption emission of fatty acids from fatty tissues, permanent loss of BG decline to 76.6 ± 3.7 mg/dL and permanent loss of physiological signals of hunger. A healthy, non-diabetic life may be recovered by a painless loss of 20% body weight (No fats, no carbohydrates) and may be maintained by implementing IHMP at reappearance of hunger sensations. This means accurate energy intake planning instead of hunger endurance.
Hilal Kılınç
Dokuz Eylül University, Turkey
Title: The new steroids from Silene Montbretiana
Time : 14:15-14:40

Biography:
Hilal Kilinç has completed her PhD from Ege University and has received scholarship from the scientific and technological research council of Turkey for 4 months to study at Salerno University under the supervision of Professor Sonia Piacente. Her studies focused on isolation, purification and structural elucidation of secondary metabolites.
Abstract:
Silene genus (Caryophyllaceae) is represented by 119 species, wild and cultivated, in Middle and East Anatolia, Azerbaijan, Iran and Iraq. Due to its wide range of biological and pharmacological effects, Silene species are used as herbal medicine. S. gonosperma, S. morcrooftiana W., S. edgeworthii, S. chlorifolia, S. acaulis, S. floscuculi L. and S. vulgaris have been used in the treatment of eye and skin problems, dysentery, inflammation, colic, malaria and stomach pains, urinary infection, respectively. S. vulgaris has been used for its sedative effect and as an anti-toxic agent. Modern biological studies have shown that Silene species exhibit a wide range of biological actions. It has been reported that S. armeria L. showed moderate antifungal activity against all plant pathogens. S. swertiifolia and S. spergulifolia had a high antibacterial activity against gram negative and positive bacteria. S. montbretiana was collected from Malatya, Turkey in 2010. Air-dried and powdered whole plant material was extracted with MeOH. After filtration, the residue was dissolved in water and then partitioned n-BuOH saturated with H2O. The n-BuOH phase was fractioned over RP-VLC to give eight main fractions. Fractions were subjected to open column chromatography by using normal and reverse phase silica gel as adsorbents. This is the first study that describes the isolation and identification of secondary metabolites from S. montbretiana. In this study, two new steroids (SM 4, SM 16) 2β,3β,14α,20S,25- pentahydroxy-cholest 7-en-6-one, 3-O-β-D-glucopyranosyl,25- O-β-D-glucopyranosyl-3β,25- dihydroxycholest-7-en-6-one, along with eight known compounds (six steroids, one flavonoid, and one cerebroside) were isolated from the methanolic extract. Their structures were elucidated by extensive spectroscopic methods including 1D- and 2D-NMR techniques as well as ESIMS and HRMS analyses.
Acknowledgement
This work is supported by TÜBITAK (114Z226) and Ege University Research Foundation (2012-Fen-047).
Recent Publications
1. Davis, P.H., 1967, Flora of Turkey and East Aegean Islands, Edinburgh University Press, Edinburgh.
2. Kayani , S. et al. 2015, J Ethnopharmacol,164:186-202.
3. Özdemir, E. and Alpınar, K., 2015, J Ethnopharmacol, 166:53-65
4. Moerman, D., 1998, Native American Ethnobotany, Timber Press, Oregon.
5. Leto, C. et al. 2013, J Ethnopharmacol, 146:90-112
6. Ballero, J. and Fresu, I., 1993, Fitoterapia, 64: 141-150.
7. Golovko, V.A. and Bushneva, O.A., 2007, B Exp Biol Med, 143:284-286
8. Bajpai, V.K. et al. 2008, Bioresource Technol, 99:8903-8908.
9. Karamian, R. and Ghasemlou, F., 2013, Int J Agric Crop Sci, 5:305-312.
Neeraj Tandon
Indian Council of Medical Research, India
Title: Therapeutic important medicinal plants and way forward
Time : 14:40-15:05

Biography:
Neeraj Tandon was a scientist g and head in Indian council of medical research, India
Abstract:
Over the last few decades alternative medicines, which are essentially plant based, have experienced a remarkable and steady increase all over the world. India with its rich biodiversity and tradition of use of herbal drugs in health care, holds tremendous opportunity for growth in a multibillion global trade, particularly in the herbal area, which has vast potential for developing multiple products for nutrition and prevention and cure of diseases. Knowledge based value addition would mean exporting value added products rather than merely the raw material, besides leading to wider acceptance of Indian plant based drugs.While herbal medicine can potentially contribute to the advancement of healthcare, many major challenges must be overcome prior to the successful integration of herbal remedies into mainstream medicine. It is a time to revisit plants with an objective of developing multicomponent botanical therapies (MCBT) with the full understanding of Systems Biology in order to develop safer and efficacious drugs. Medicinal and aromatic plants (MAPs) act as a well spring of traditional medicines, herbal drugs, nutraceuticals, new chemical entities as drugs and drug intermediates, sweetners, flavour, fragrances, insecticides, natural cosmetics and a number of health care realted products. No doubt, the MAPs have substantially and significantly contributed to the drug armanetarium of the modern Allopathic system of medicine and towards traditional medicines. Bioresource rich nations will be exploring untapped bioresources to meet the demands. India is blessed with rich biodiversity of bioresources and tremendous traditional knowledge base for healthcare products. At present nearly four billion people of the world use plant derived healthcare products. Each plant part used in our traditional systems of medicine (Ayurveda, Unani & Siddha) is a complex mixture of many primary and secondary metabolites. Developing MCBT will provide much safer and efficacious drugs. Plant drugs sound an answer for prioritized diseases such as protozoal diseases (trypanosomiasis, malaria, filaria, leishmania, amoebiasis), viral diseases (Dengue, herpes, Aids, bird flue), metabolic disorders (inflammation, arthritis, diabetes, hypercholestrolaemia), diseases of less known etiology (cancer, muscular dystrophy, Parkinson’s diseases), cardiovascular and central nervous system disorders and self-inflicted diseases (obesity, depression), HIV/AID etc. Standardisation of raw materials has been one of the major impediments in wider acceptance of herbal drugs. In an effort to address this issue monographs on Quality Standards of important medicinal plants used by the industry are being developed by Indian Council of Medical Research on the basis of WHO guidelines for widely used raw materials involving laboratories of reputed institutions across the country to generate requisite data as per prescribed format. The monographs translate practically generated knowledge into direct utility for the Indian Drug Industry engaged in production of plant based drugs and for the Pharmacopoieal Commissions in India & abroad for developing official quality standards on plant based drugs Special emphasis has been laid on chromatographic finger printing of the extracts and assay using phytochemical reference standards as one of the parameters of identity, purity and quality under this programme. The endeavour has yielded very fruitful results evidenced by the publication of 14 volumes of Quality Standards of Indian Medicinal Plants containing 484 plants. The work continues to progress on remaining potential plants required by the industry. Another programme has been initiated on the isolation of Phytochemical Reference Standards(PRS), a key factor in standardization from selected medicinal plants. The procedure of isolation have been optimised and characterized both on the basis of chromatography and spectroscopy for the benefit of these interested in standardizing drugs. Ninety PRS have been isolated and monographs have been prepared and published as three volumes of Phytochemical Reference Standards of Selected Indian Medicinal plants. The samples of these marker compounds are stored as a repository. The work is continuing on other important PRS. Quality standards for the medicinal plants used in India are absolutely necessary for the drugs and formulations produced from them to be of adequate quality, safety and efficacy for their wider acceptance
William G Whitford
GE Healthcare, USA
Title: Continuous biomanufacturing of extracellular vesicles
Time : 15:05-15:30

Biography:
William G Whitford is Strategic Solutions Leader, GE Healthcare in Logan, UT with over 20 years of experience in biotechnology product and process development. He joined the company as an R&D Leader developing products supporting protein biological and vaccine production in mammalian and invertebrate cell lines. Products he has commercialized include defined hybridoma and perfusion cell culture media, fed-batch supplements and aqueous lipid dispersions. He has published over 250 articles, book chapters and patents in the bioproduction arena.
Abstract:
Interest in microvesicles, exosomes and oncosomes is growing. Applications include 1) Vectors of research or therapeutic cargo, 2) Agents of intercellular communication from stems cells to terminally differentiated tissue to the entire microbiome and 3) Support of clinical diagnostics. There are ongoing efforts to standardize clinically applied vesicle assays and therapeutic cargo vehicles. Reference materials, controls, and performance standards need to be defined for quality assurance in such applications. Sponsors often have their choice of cell platforms, production formats and culture modes for vesicle product and process development. However, commercial success can be dependent upon the discovery of scalable technologies that can produce very large amounts of sufficiently pure and consistent vesicles in a robust, compliant and cost-effective manner in a cGMP environment. In biopharmaceuticals, continuous biomanufacturing promises heightened process flexibility and a reduction in product microheterogeneity; construction costs and schedule extent; utilities requirement; manufacturing suite area and classification. The value of single-use implemented and continuous biomanufacturing methods will be reviewed
Sánchez-Labastida Luis Angel
National Polytechnic Institute, México
Title: α,β-unsaturated compounds derived from arylamines as possible new treatment against leukemia
Time : 15:30-15:55

Biography:
Sánchez-Labastida Luis Angel is a third year Medical student at Escuela Superior de Medicina of Instituto Politécnico Nacional. In 2013, he started working in the Department of Molecular Biology, and in 2015, in the Biochemistry department, searching for possible treatments against cancer.
Abstract:
L5178 cells are an experimental lymphocytic leukemia in mice, associated with hyperplasia of the lymphoid tissues and a high number of circulating malignant lymphocytes and lymphoblast, this cellular line was used to evaluate the activity of a maleimide and a maleimide of phenethylamine, as a possible new treatment for leukemia. Previous studies have shown that α, β-unsaturated compounds have important pharmacological properties, as an anti-tumoral activity, this through reducing glutathione levels and increasing oxidative stress, causing cytotoxicity, reduced viability, and death by apoptosis. As the first step, α, β-unsaturated compounds were designed from phenylethylamine, the two best candidates were selected. New green synthesis techniques were designed for both compounds and were synthesized, the chemical structure and purity were confirmed by NMR 1H and 13C, mass spectrometry and IR. The compounds were tested in an in vitro experiment with L5178-Y cell culture (50,000 cells approximately per well), treated with the compounds at concentrations of 1x10-3 to 1x10-9 M in both cases. Maleimide derivative showed an activity on cells in concentrations of 1x10-6 to 1x10-4 M, evidenced by the MTT assay at 24 and 48 hours, after that, the field was opened at a concentration between 1x10-6 to 10x10-6 M, and an EC50 of 5x10-6 was obtained. For the case of maleimide, an activity was observed at 1x10-3 to 1x10-5 M, and the open field between 1x10-5 to 1x10-4 M showed an EC50 of 3x10-5 M. The experiment results lead us the possibility to evaluate these compounds in an in vivo models such as survival experiments or LD50 in mice.
Szu-Han Chen
National Taiwan University, Taiwan
Title: Synthesis of Oseltamvir Using Diels–Alder Reaction of 1,3-Butadiene Bearing 2-Carboxy and 4-Alkoxy Substituenes

Biography:
Szu-Han Chen was born in Taipei, Taiwan, in 1985. She received her B.S. in chemistry from Fu Jen Catholic University in 2008 and her M.S. degree in chemistry from National Taiwan Normal University in 2010. She is a Ph.D. student in the Department of Chemistry, National Taiwan University. Her research interests are in total synthesis of drug molecules as well as medicinal and biological chemistry.
Abstract:
Diels–Alder reactions are particularly useful for the total synthesis of pharmacologically active compounds and natural products. Not only does this strategy construct two new C–C σ-bonds in one step, but it also forms a cyclohexene system with good regio- and stereoselectivity http://medicinalchemistry.pharmaceuticalconferences.com/europe/up to four contiguous stereocenters. Using heteroatom-substituted electron-rich dienes, such as Danishefsky’s diene, usually promotes the normal electron-demand Diels–Alder reactions in highly regioselective fashion.
Tamiflu, the phosphate salt of oseltamivir, is a popular anti-influenza drug in clinical use. Diels–Alder reactions using 1,3-butadiene, 1-timethylsilyoxy-1,3-butadiene, furan, N-Boc-pyrrole and 1-Cbz-1,2-dihydropyridine have been successfully applied to react with appropriate dienophiles for construction of the cyclohexene core structure of oseltamivir.
We synthesized a novel diene precursor bearing both 3-pentoxy and ester groups. Dimerization of this diene was overcome by trapping it in situ using activated alkenes as the dienophiles. Inspired by Shibasaki’s work, we successfully synthesized a racemic mixture of oseltamivir via a sequence of reactions that comprise acyl azide formation and Curtius rearrangement. The synthesis of optically active oseltamivir via asymmetric Diels–Alder reaction is currently under investigation.
Wei-Hsin Hsu
National Taiwan University, Taiwan
Title: Design and Synthesis of Sugar Phosphates for Inhibition of Tuberculosis Maltosytransferase

Biography:
Wei-Hsin Hsu received her B.S. from Department of Applied Chemisrty, Nartional Chiao Tung University in Taiwan. She is a M.S. student in the Department of Chemisrty, National Taiwan University.
Abstract:
Tuberculosis, a disease caused by bacterial pathogens Mycobacterium tuberculosis, was regarded as under control in the past decades. However, the multi-drug-resistant tuberculosis (MDR-TB) and extensively-drug-resistant tuberculosis (XDR-TB) have emerged to become a serious global health crisis. In 2015, there were an estimated 10.4 million new TB cases worldwide, including nearly a half million cases of MDR-TB. 1 Bedaquiline and delamanid are the effective drugs for treatment of MDR-TB. However, development of new drugs by targeting different TB proteins is still needed for treatment of the MDR-TB and XDR-TB patients.
GlgE is a maltosyltransferase that uses maltose-1-phosphate as the substrate. GlgE involves in a four-step pathway for the production of α-glucan from trehalose, an essential process for mycobacterial survival. Inhibition of GlgE will cause accumulation of maltose-1-phosphate,2 and trigger the self-poisoning of M. tuberculosis. GlgE becomes an appealing drug target according to the toxic effect and synthetic lethal pathway. As no effective GlgE inhibitor has been discovered, we thus designed and synthesized some potential GlgE inhibitors by mimicking the structure maltose-1-phosphate, the GlgE substrate.
- Synthetic Organic Chemistry|Anticancer Agents in Medicinal Chemistry|Bioorganic and Medicinal Chemistry

Chair
Maia Merlani
Tbilisi State Medical University, Georgia

Co-Chair
John Yuen
The Hong Kong Polytechnic University , Hong Kong
Session Introduction
Maia Merlani
Tbilisi State Medical University, Georgia
Title: 5-Steroidal hydrazones: Synthesis and biological activity
Time : 12:15-12:40

Biography:
Maia Merlani has completed her PhD from Tbilisi State University. She is a senior research scientist at Tbilisi State Medical University. Her field of interest is a chemistry and synthesis of biologically active compounds. She is the author of more than 50 papers in reputed journals and presentations at 60 international scientific conferences. She was granted Georgian Presidential scholarship for young scientists (1997), NATO scholarship (2002, 2003) and Matsumae International foundation scholarship (2013). She is a member of organizing committee of several international conferences in the field of organic and pharmaceutical Chemistry.
Abstract:
Several new 5α-steroidal hydrazones have been synthesized and examined for their biological activities. The starting compound for the synthesis was tigogenin, isolated from the plant Jucca gloriosa (family Liliaceae) introduced in Georgia. The condensation of 3β-hydroxy-5α-pregn-16-en-20-one with isonicotinoylhydrazide or p-nitrophenylhydrazine at room temperature in ethanol in the presence of a catalytic amount of glacial acetic acid gives corresponding hydrazone, whereas treatment of the same ketones with phenylhydrazine, p-bromophenyl-, p-chlorophenyl- or p-methylphenylydrazine in the same conditions produces just product of their intramolecular cyclyzation – pyrazolines. Obtained steroidal isonicotinoylhydrazones readily cyclizes on heating to 5α-androstano- [17,16-d]-pyrazolines, whereas in the same conditions cyclization of p-nitrophenylhydrazones did not proceed. It can be explained by the effect of electron withdrawing substituent at the amine nitrogen atom of the hydrazone fragment of p-nitrophenylydrazones, which prevents process of their cyclization. Reaction of epiandrosterone and 5α-androstan-3,17-dione with isonicotinoylhydrazide, m-nitrobenzhydrazide or m-bromobenzhydrazide yields corresponding hydrazones. All synthesized hydrazones and pyrazolines have been examined for their biological activities. Isonicotinoylhydrazone of epiandrosterone revealed high antituberculousis activity against M.tuberculosis H37Rv and can be considered as promising antituberculosis agent. Bis-hydrazones of 5α-androstan-3,17-dione showed high anticancer and anti-HIV activity.
Abdulmajeed S H AlSamarrai
Department of Chemistry, University of Samarra, Iraq
Title: Synthesis and characterization of 2-((4R,4aR,5aS,6S)-1,3-dioxo3m3a,4,4a,5,5a6,6a-octahydro-4,6- ethenocyclopropa[f]isoindol-2(1H)-yl)-N-(substituted phenyl) acetamides anticipated to have HIV- 1 activity
Time : 12:40-13:05

Biography:
Abdulmajeed S H AlSamarrai was awarded a BSc in chemistry by the Mosul University of Iraq and MSc in analytical chemistry , by Baghdad University of Iraq and was awarded a PhD in organic chemistry by university of Birmingham/ UK in 1984. He is an assistant professor, department of chemistry, Tikrit university , Iraq (1991-2001). Then since 2001, he is a faculty member in the department of chemistry at the University of Samarra, Iraq and he is working as a professor of organic chemistry. Dr. ALSamarrai research interests are on organic synthesis of heterocyclic compounds and has published about 30 papers in reputed journals.
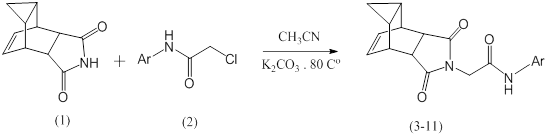
Abstract:
This work includes the synthesis of some new 2-((4R,4aR,5aS,6S)1,3-dioxo3,3a,4,4a,5,5a6,6a-octahydro-4,6-ethenocyclopropa[f]isoindol-2(1H)yl)-N-(substituted phenyl) acetamides (3-11) by reaction of (4R,4aR,5aS,6S)-1,3-dioxo3,3a,4,4a,5,5a,6,6aoctahydro4,6ethenocyclopropa[f]isoindole1,3(2H,3aH)-dione (1) with 2-chloro-N-(substituted phenyl) acetamides (2) in the presences of potassium carbonate in refluxing acetonitrile as a solvent. These compounds have been identified by spectroscopic methods including, IR, 1H-NMR, 13C-NMR and elemental analysis (CHN).
Hasnah Osman
Universiti Sains Malaysia, Malaysia
Title: Identification of thiadiazinyl-coumarin derivatives as dengue virus-2 NS2B/NS3 protease inhibitors
Time : 14:20-14-45

Biography:
Hasnah Osman has graduated with bachelor of science in chemistry from Universiti Sains Malaysia in 1985. She obtained her Master’s degree in 1998 and was appointed as a Lecturer in the year 2000. She completed her PhD in the field of synthetic organic chemistry from University of Otago, New Zealand in 2005. She was promoted to Senior Lecturer in the year 2007, associate professor in 2009 and then became a professor in the year 2014. She is a Member of the Malaysian Institute of Chemistry (since 1998), the American Chemical Society (since 2008) and the Royal Society of Chemistry (since 2009). Currently, she is an ExCo member of the Malaysia Natural Product Society and a Secretary of the Malaysian Ionic Liquid Society. In term of research, she has published more than 150 papers in reputed journals and has been serving as an Editorial Board Member of repute.
Abstract:
Dengue is a dreadful arboviral disease which has become a major public health concern especially in tropical and sub-tropical regions. Despite several reports of peptide based dengue inhibitors, recently the development of small molecules against dengue has gained tremendous momentum. Moreover, selecting an accurate drug target is a critical task in finding agents with a particular biological activity that is anticipated to have therapeutic efficacy. Thus, role played by the NS2B/NS3 protein in the dengue virus life cycle is envisaged to form a potential attractive target for dengue inhibitors drug design. Coumarin analogues have received a considerable attention as a lead structure for the discovery of antiviral agents. Therefore, coumarin based thiadiazinyl hydrazones were designed as potential NS2B/NS3 protease inhibitors. Molecular docking studies provided insights into the binding modes of the inhibitors. All compounds were observed to have good interactions with the active site residues.
Yoko Matsumoto
Sojo University, Japan
Title: Therapeutic effects of trehalose liposomes against carcinoma along with apoptosis in vitro and in vivo
Time : 14:45-15:10

Biography:
Yoko matsumoto is a professor in the department of life sciences at Sojo University, Japan. She received her PhD in pharmacy from Kyushu University, Japan. She was a visiting researcher in Colorado University. She has received outstanding female researcher award from the society of chemical engineering, Japan. She is one of the directors for Japan nanomedicine society and councilor for japanese association for molecular target therapy of cancer. Her current research interest focuses on trehalose liposomes for therapeutic applications. She has published more than 120 papers in reputed journals.
Abstract:
Trehalose stabilizes membranes and proteins in cells most likely by hydrogen bonding. Trehalose liposomes (DMTre) composed of DMPC and trehalose micelles have been produced. Hydrodynamic diameter (dhy) of DMTre composed of 30 mol% DMPC and 70 mol% TreC14 was 100 nm with single and narrow range of size distribution, which was preserved for a period remaining stable for more than one month. The thickness of the fixed aqueous layer (TFAL) of DMTreCn was evaluated from the zeta potential and increase in TFAL values of DMTreCn was obtained in a dose-dependent manner. The TFAL values for DMTreCn were larger than that of DMPC liposomes. The remarkable inhibitory effects of DMTre on the growth of human colon, gastric, hepatocellular and lymphoblastic carcinoma cells have been reported. In this study, the inhibitory effects of DMTre on the growth of lung carcinoma (A-549) cells were examined in vitro and in vivo. DMTre inhibited the growth of A-549 cells leading to apoptosis. The activation of caspase-3, 8, and 9 was obtained for A-549 cells treated with DMTre. The suppression of tumor weight of xenograft mice model of carcinoma treated with DMTre was obtained. To investigate induction of apoptosis against tumor, the tissue section of tumor in
xenograft mice model of carcinoma after the treatment with DMTre was dyed and observed using microscope. Many apoptotic brown color cells in the tissue section of tumor was observed, indicating that DMTre could induce apoptosis in tumor cells against xenograft mice model of carcinoma.
John W M Yuen
The Hong Kong Polytechnic University, Hong Kong
Title: Quantification of some medical preparations of the plant origin
Time : 15:10-15:35

Biography:
John W M Yuen is currently an Associate Professor from the School of Nursing of the Hong Kong Polytechnic University. He is a biomedical scientist who has
completed his PhD in 2007, with a focus on cancer and immunology in the field of Urology. His research team conducts different types of research by adopting a
wide range of methodologies from exploratory cross-sectional/cohort design and in vitro laboratory experiments to in vivo trails on animals and humans.
Abstract:
Immunotherapeutic effects of the ethanol extract of Ganoderma lucidum (GLe) were compared against the conventional immunobladder® Bacillus Calmette-Guérin (BCG) in terms of cytotoxicity, cell cycle analysis and cytokine genes expression, in vitro. In conjunction with the intravesical study using the orthotopic MB49/C57 mice model, the murine urothelial carcinoma MB49 cell line was used for experiments. In agreement with the previous findings, GLe was demonstrated to exhibit G2/M phase cell arrest. On the other hand, dose-dependent cytotoxicity was demonstrated by both GLe and BCG as measured by the lactate dehydrogenase (LDH) assay; however, GLe concentrations ranged from 40 to 100 μg/ml killed 24.7-88.1% of the MB49 cells, which was superior to the 250-1000 μg/ml of BCG that killed 7.6-19.6%. Such cytotoxic effects were also shown to be inter-correlated with the expression of several cytokine genes, which are known to be important for anticancer. Although both GLe and BCG were shown to be active in inducing the interleukin(IL)-6, IL-12b and interferon-gamma (IFN-γ), dose-dependent inductions were only demonstrated by the range of GLe concentrations being tested. Particularly, the induction of IFN-γ gene was denominated by GLe up to 4-folded, as compared with the 1.5-folded increase by BCG. Basic research on immunobladder® BCG is limited and given that IFN-γ is wellevidenced for its anticancer effects, results here in speculated GLe could be an immunotherapeutic agent superior to the BCG by exerting stronger cytotoxic effects via a pathway involving IFN-γ and other molecules. The in vivo effects of GLe are currently being examined in animals.

Biography:
Abdulmajeed S H AlSamarrai was awarded a BSc in chemistry by the Mosul University of Iraq and MSc in analytical chemistry , by Baghdad University of Iraq and was awarded a PhD in organic chemistry by university of Birmingham/ UK in 1984. He is an assistant professor, department of chemistry, Tikrit university , Iraq (1991-2001). Then since 2001, he is a faculty member in the department of chemistry at the University of Samarra, Iraq and he is working as a professor of organic chemistry. Dr. ALSamarrai research interests are on organic synthesis of heterocyclic compounds and has published about 30 papers in reputed journals
Abstract:
This work includes the synthesis of some new 2-((4R,4aR,5aS,6S)-1,3-dioxo3,3a,4,4a,5,5a6,6a-octahydro-4,6-ethenocyclopropa[f]isoindol-2(1H)-yl)-N-(substituted phenyl) acetamides (3-11) by reaction of- (4R,4aR,5aS,6S)-1,3-dioxo3,3a,4,4a,5,5a6,6a-octahydro-4,6-ethenocyclopropa[f]isoindole1,3-(2H,3aH)-dione (1) with 2-chloro-N-(substituted phenyl) acetamides (2) in the presences of potassium carbonate in refluxing acetonitrile as a solvent.These compounds have been identified by spectroscopic Methods including, IR, 1H-NMR,13-NMR and elemental analysis (CHN).

Biography:
Zhongli Gao, Ph. D. is currently a Sr. Principal Scientist at Sanofi. He received his Ph. D. in Organic Chemistry at City University of New York in 1993. Upon graduation, he carried out his postdoctoral research on the total synthesis of Spinosyn A supervised by Professor Paquette at Ohio State University. He joined Hoechst Pharmaceuticals, one of the predecessor companies of Sanofi in 1995. He has worked on a broad range of disease areas in CNS, respiratory, inflammation, oncology, and rare disease involving GPCRs, proteases, enzymes, kinases, ion channels and transporters. He has led many projects in advancing compounds into clinical and preclinical or go/no-go decisions. In particular, his team has successfully transitioned SAR152954, a H3 receptor antagonist for Cognitive Impairment, and AVE8923, an orally active β-tryptase inhibitor for Asthma, into clinical development. He held 29 patents/ patent applications and 20+ peer reviewed publications.
Abstract:
Scaffold hopping is a technique that generates compounds containing a topologically different scaffold from the parent compound, but with similar or improved activity and other properties. This can be done intellectually by medicinal chemists or by computational algorithm. The technique is based on topological pharmacophore models developed from SAR of the current lead. In scaffold hopping, medicinal chemist’s insights into pharmacophore and SAR are crucial in this iterative process. Scaffold hopping can be used to generate new chemical entity; overcome patent or other limitations for the current leads; generate differentiated series of chemical matters. Common approaches include heterocycle replacements, bonds formation and cleavage, and topology-based hopping. This presentation will describe medicinal chemistry of H3 receptor antagonist and tryptase inhibitor programs via using scaffold hopping strategy to generate multi-genesis of chemical leads. Through optimization of the leads, clinical candidates were identified. The profile of the candidates and in vivo effects in disease animal models will also be briefly discussed.
Recent Publications (minimum 5)
- Sun H, Tawa G, Wallqvist A (2012) Classification of scaffold-hopping approaches. Drug Discovery Today 17: 310-324
- Hu Y, Stumpfe D, Bajorath J (2016) Recent Advances in Scaffold Hopping. J. Med. Chem.
- Gao Z, Hurst WJ, Hall D, Hartung R, Reynolds W, Kang J, Nagorny R, Hendrix JA, George PG (2015) Design and synthesis of a novel series of histamine H3 receptor antagonists through a scaffold hopping strategy. Bioorganic & Medicinal Chemistry. 23: 429–438
- Gao Z, Hurst WJ, et al., (2013) Synthesis, characterization, and biological assessment of the four stereoisomers of the H3 receptor antagonist 5-fluoro-2-methyl-N-[2-methyl-4-(2-methyl[1,3′]bipyrrolidinyl-1′-yl) phenyl] benzamide. Bioorganic & Medicinal Chemistry Letters. 23:4044–4047.
- Gao Z, Hurst WJ, et al., (2013) Identification and profiling of 3,5-dimethyl-isoxazole-4-carboxylic acid [2-methyl-4-((2S,3’S)-2-methyl-[1,30]bipyrrolidinyl-10-yl)phenyl] amide as histamine H3 receptor antagonist for the treatment of depression. Bioorganic & Medicinal Chemistry Letters. 23:6269–6273.
- Gao Z, Hurst WJ, et al., (2013) Discovery of a potent, selective, and orally bioavailable histamine H3 receptor antagonist SAR110068 for the treatment of sleep–wake disorders. Bioorganic & Medicinal Chemistry Letters. 23:6141–6145.
Jana Sopkova-de Oliveira Santos
University of Caen Normandy, France
Title: β-Strand Mimicry: Exploring Oligothienylpyridine Foldamers
Biography:
Jana Sopkova-de Oliveira Santos has completed his PhD at the age of 26 years from University Paris XI (Orsay)and University of Charles (Prague). Since 2012, she is a Professor of General Chemistry and Biophysic at University of Caen Normandy. She has published more than 110 papers in reputed journals.
Abstract:
Protein–protein interactions (PPIs) are involved in many cellular processes; consequently, the discovery of small molecules as modulators of PPIs has become a challenge in medicinal chemistry. Structural mimetics of protein secondary structures could maintain or restore biological functions and should possess biological activity. Actually, the most challenging classes of PPIs are those mediated by β-sheet, which are implicated in a number of diseases. Only a few β-strand mimics have been published to date. This study presents an evaluation of oligothienylpyridyl scaffolds in view of their ability for β-strand mimicry.
We have observed that a coplanar arrangement in thienylpyridyl systems can be obtained in several different ways. The presence of a nitogen and sulfur atom in the junction vicinity introduces a coplanar arrangement as well as the presence on the nitrogen atom in the non-ortho-substituted systems. The introduction of an ortho substituent in a system with a nitrogen atom in the junction vicinity perturbs the two rings somewhat, but the system can achieve the coplanar arrangement, because the energy barrier is very low. The same behavior was observed in a non-ortho-substituted biaryl with only a sulfur atom in the junction vicinity. The X-ray structures showed that the compounds have a tendency to adopt a nearly coplanar conformation and the positions of methyl substituents coincide well with those of i, i + 2nd or i, i + 4th β-strand side chains. Therefore, the thienylpyridine scaffold opens the way to produce coplanar compounds mimicking β-strand side-chain distributions.
Fabiola Porta
University of Basel, Switzerland
Title: Ezyme triggered nanomaterial for cancer therapy

Biography:
Dr. Fabiola Porta has studied Medicinal Chemistry and Pharmaceutical Technology in Milan at Universita’ degli studi, where she obtained her Master degree in Pharmacy in 2008. She then moved to the Leiden Institute of Chemistry where she graduated, in 2012, in chemistry with a special focus in nanoparticles synthesis and characterization. After the completion of her PhD she started to work as PostDoc in several institutions in Switzerland as: FHNW and University of Basel. Recently, she joined the group of Biopharmacy where she is developing-self assembled nanoparticles for cancer therapy. Dr. Porta expertise is in the design and development of nanovehicles for cancer therapy with aparticular focus on targeted nanocarriers for breast cancer therapy.
Abstract:
Polymer vesicles, are attracting much attention as alternative nanodelivery system to implement drug targeting strategies. Polymersomes have several interesting features. For instance, ease of chemical modification of the polymer chains can be used to modulate their tissue specificity and organ distribution. A wide variety of polymers is available, however a good candidate for pharmaceutical formulations is the di-block copolymer poly(dimethylsiloxane)-b-poly(2-methyloxazoline) (PDMS-PMOXA). This polymer is formed by two subunits which are FDA approved for the development of novel nanomaterials with a potential human use. In our work we are developing a responsive nanomaterial for the treatment of breast cancer. In order to develop a targeted delivery system a very good understanding of the cancer biology is necessary. In particular cancer biomarker are of particular interest due to their specificity for cancer cells. The enzyme family of cathepsins are highly expressed in certain type of cancers as breast tumor. Moreover, they are responsible for the degradation of proteins in the lysosomes. The elevate expression of these enzymes in tumors is an evidence of the increased metabolism of cancer cells. For this reason, these enzymes are a very interesting target for the development of a novel nanomaterial. In this work we are presenting a peptide cross linked polymeric nanoparticle with the main goal to encapsulate anticancer compounds and to release them only upon activation of the system by cathpesin B.
Recent Publications
- F. Porta and Alexander Kros, Colloidosomes as single implantable beads for the in vivo delivery of hydrophobic drugs, Particles & Particles Systems Characterization, 2013, 30, 606-613
- P. Nadrah, F. Porta, O. Planinšek, A. Kros, M. GaberšÄek , Poly(propylene imine) dendrimer caps on mesoporous silica nanoparticles for redox-responsive release: smaller is better, PCCP, 2013, 15, 10740-10748
- D. Witzigmann, S. Sieber, F. Porta, P. Grossen, A. Bieri, N. Strelnikova, T. Pfohl, C. Prescianotto-Baschong and J. Huwyler, Formation of lipid and polymer based gold nanohybrids using a nanoreactor approach, RCS Adv., 2015, 5, 74320
- D. Gliesche, J. Hussner, D. Witzigmann, F. Porta, T. Glatter, A. Schmidt, J. Huwyler, H. Meyer zu Schwabedissen, Secreted Matrix Metalloproteinase-9 of proliferating smooth muscle cells as a trigger for drug release from stent surface polymers in coronary arteries, Mol. Pharm., 2016, 13 (7), 2290-2300,
- D. Witzigmann, P. Detampel, F. Porta, J. Huwyler, Isolation of multiantennary N-glycans from glycoproteins for hepatocyte specific targeting via the asialoglycoprotein receptor, RCS Adv., 2016, 6, 97636-97640,
Hamid Irannejad
Mazandaran University of Medical Sciences, Sari, IRAN
Title: Synthesis and neuroprotective activity of novel 5,6-Diaryl-1,2,4-triazine derivatives with ethyl acetate moiety against H2O2 and Aβ-induced neurotoxicity

Biography:
Hamid Irannejad has completed his Pharm.D at Kerman University of Medical Sciences and PhD at Tehran University of Medical Sciences, IRAN. Postdoctoral studies was accomplished at University of Siena, Italy, under the supervision of Prof. Maurizio Botta. Currently, he is serving as an assistant professor at Mazandaran University of Medical Sciences. He has published nearly 20 papers in reputed journals in the field of medicinal chemistry.
Abstract:
Alzheimer’s disease (AD) is a neuropathologic disorder characterized by intracellular neurofibrillary tangles and amyloid aggregates in the CNS. In recent years numerous approaches have been used to combat AD like small molecule inhibitors of Aβ aggregation, anti-inflammatory agents, cholinesterase, β- and γ-secretase.
Herein, we report synthesis of some 5,6-diaryl-1,2,4-triazines 3a-f and 8a-e as potential agents for treatment of AD. We evaluated them against both H2O2 and β-amyloid induced toxicity in PC-12 and SH-SY5Y cells and the extent of cell viability and apoptosis were assessed.
The synthesis of compounds (3a-f) was started by 1,2-diketones, in which triazine ring closure was performed by thiosemicarbazide and alkylation by ethyl chloroacetate to afford compounds 3a-f.
synthetic route for compounds 8a-e was started by an acylation reaction of anisole with phenylacetic acid derivatives. The oximation in the alpha position of carbonyl group was performed by use of sodium methoxide and butylnitrite. The next two steps, were performed similarly to afford final compounds 8a-e.
All compounds showed significant neuroprotective activity with EC50 values ranging from 14-30 µM. Most compounds could increase cell viability compared to amyloid treated group.
Surprisingly, 3-thioxo-1,2,4-triazin-2(3H)-yl)acetate derivative 8e was the most potent compound in both tests with EC50 of 14 µM and could increase 40% of cell viability revealed by cytometric analysis with Annexin V/PI staining. It was also shown that 8e has more neuroprotective activity than Quercetin. Morphologic evaluation of cells by DAPI staining and TUNEL assay showed the effectiveness of this compound to improve neurite outgrowth in neuronal cells.
Yun Lu
Southern Illinois University Edwardsville, USA
Title: Mapping the Vibrational Transition-State Conformational Change in Enzymes for Drug Design

Biography:
Yun Lu received his Ph.D. degree in Organic Chemistry in 1996 from Nankai University, China. He did his postdoctoral work in Utah State University with Professor Vernon D. Parker and Wayne State University with Professor Martin Newcomb. His research focuses on the field of physical organic chemistry studying the mechanism of organic reactions and the transition state structures. He is now a professor in the Department of Chemistry at Southern Illinois University Edwardsville, USA. He has published nearly 50 papers in reputed journals.
Abstract:
About a quarter of the current registered pharmaceutical drugs are enzyme inhibitors. Many of them are the enzyme transition-state (TS) analogues that are designed largely on the basis of the crystal structure of the stable “frozen” TS analogues or the “still” TS structures from the kinetic studies. Recent decade has, however, witnessed much of the role of the enzyme dynamics in catalysis. Protein vibrations with different time scales have been proposed to assist various processes of the complex enzymatic reactions, including the bond-formation and - cleavage in the active site. The latter suggests a vibrational TS structure on the reaction coordinate, coupled with the local fast motions of enzyme. While study of the vibrational TS that involves the fast fluctuations of the reaction distance is still in its infancy, it would be worthy to consider the concept in design of what may come to be more effective inhibitors and more successful drugs. E.g., a successful inhibitor may be that can also interrupt the TS/enzyme coupled vibrations. In this paper, we present a method to gain the different TS structures at different donor-acceptor distance (DAD) in both solution and enzymatic H-transfer reactions. We use secondary (2°) kinetic isotope effect (KIE) as a structural descriptor. We determine the 2° KIEs and thus TS structures for hydride- and deuteride-tunneling processes that have different DADs. Information about the DAD-dependent TS structures in enzymes would help track the path of the vibrational TS conformational changes. This information would be useful for drug design.
S.I. Voychuk
Zabolotny Institute of Microbiology and Virology of NASU, Ukraine
Title: Morphological and structural features of adenovirus particles by reacting with bacterial cell

Biography:
Serhiy Voychuk has completed his PhD at the age of 26 years at Zabolotny Institute of Microbiology and Virology of NASU and continued his work in this institute. He is a senior researcher and a supervisor of a team of researchers with a broad spectrum of scientific interests in microbiology, virology, biotechnology and medicine. He is a coauthor of more than 40 papers
Abstract:
Antiviral drugs mainly targeted onto prevention of viral synthesis within the host cells or elimination of it enters into the cell through induction of natural defense mechanisms, among which are immune response and interferon synthesis. Recently, it was suggested that human microbiota may play a role in virus infection. Human viruses did not infect bacteria cells, but this does not exclude a possibility of bacteria participation in the virus propagation into the host cells.
An interaction of lactic acid bacteria with human adenovirus serotype5 (HAdV-C5) was studied. We used strains of bacteria some of which are normally can be found in human intestine, while others are supplied with various milk products: Lactobacillus plantarum (56 strains), Enterococcus spp. (23 strains), Leuconostoc spp. (14 strains), Lactococcuse lactis (3 strains) and Pediococcus spp. (1 strain). After 1h of interaction of viruses (VPs) with bacteria the samples were examined with electron microscopy.
In 17% of cases there were no VPs found that can suggest their total destruction, but in other cases the VPs were clearly seen performing well preserved viruses and viral proteins. The direct viral adhesion to the surface of bacteria was noticed for 23% of the strains, while in other cases the VPs were associated with extracellular matrix structures or as the free particles.
The current research is one of the first steps in understanding the role of microbiota in the virus infections development. It showed that various strains of the same bacterial species can cause opposite effects and lead both to the virus degradation or preservation and, therefore, can prevent or help virus to enter the human cells.
Daniel Ehrsam
University of Basel, Switzerland
Title: Early findings in the development of an enzymatically triggered nanoformulation

Biography:
Daniel Ehrsam studied Pharmaceutical Sciences and graduated at University of Basel in March 2014. He conducted his master thesis in research of brain ischemia pathways at Texas Tech Health Science Center under supervision of Dr. Margaret Weiss. He then worked at the Swiss Tropical and Public Health Institute on Helminth drug development (Prof. Dr. Jennifer Keiser). In August 2015 he joined the research group of Prof. Dr. Henriette E. Meyer zu Schwabedissen for his PhD studies. His research is focused on the development of an enzyme based targeted drug delivery system.
Abstract:
In order to spare healthy cells and decrease adverse effects, innovative concepts of tumor targeting aim at bringing the cytotoxic payload most selectively to tumor cells. One concept is to use enzymes overexpressed in the surrounding of proliferating cells, like the gelatinase matrix-metalloproteinase 9 (MMP-9), as a trigger for drug release.
Our aim is to synthesize and characterize self-assembling nanoformulations consisting of an MMP9-labile peptide coupled to an anti-cancer drug.
By use of bioconjugate chemistry an amphiphilic molecule containing paclitaxel and an MMP9-labile peptide was synthesized to form nanoparticles. To identify a tumor entity as a target for our novel nanoformulation we quantified expression of MMP-9 in a commercially available tissue collection by multiplex real-time PCR. Several tumor entities showed significantly increased expression comparing normal to malignant tissue. Immunohistochemistry and database analysis suggested brain tumors, particularly glioblastoma multiforme, as a tumor entity where MMP-9 could be used to trigger drug release. Established brain cancer cell lines were characterized for MMP-9 expression and activity. LN-18 and U87-MG cells were selected for in vitro characterization of the synthesized nanoformulation. In preparation of in vivo xenograft studies LN-18 and U87-MG cells were stably transfected with mKate2 and characterized for expression.
Taken together, we verified overexpression of MMP9 in glioblastoma multiforme. Commonly used brain cancer cell lines were characterized prior to in vitro studies on MMP9 triggered drug release, and preparations for in vivo xenograft studies have been finished. Further studies are warranted to fully characterize the nanoformulation and understand its effects in vivo.
- Bioorganic and Medicinal Chemistry|Drug Design and Drug Development

Chair
Nana Gorgaslidze
Tbilisi State Medical University, Georgia

Co-Chair
Alexander Heifetz
Evotec Ltd, UK
Session Introduction
Nana Gorgaslidze
Tbilisi State Medical University, Georgia
Title: Development of analytical procedure for the standardization of bromelain from the pineapple (Ananas comosus L. Merr)
Time : 16:10-16:35

Biography:
Nana Gorgaslidze has completed her PhD from Saint-Petersburg State Chemical-Pharmaceutical Academy. She is the Director of TSMU I Kutateladze Institute of Pharmacochemistry and Professor at the Department of Social and Clinical Pharmacy at Tbilisi State Medical University. She has published more than 80 papers in reputed journals.
Abstract:
In presented work the results of preliminary investigation for standardization of fruit bromelain and stem bromelain are given. Comparative characteristic of some physical-chemical properties of the sample of both species is presented. The effect of cysteine and casein concentration on hydrolysis rate, dependence of casein lysis rate on bromelain concentration and reaction duration is shown. Influence of pH on the activity of both (fruit and stem) of bromelain was studied. Optimal pH value was also established. The dependence on pH is similar in both cases. Optimal value of pH is 7.5 for stem bromelain and 8 for fruit one. Determination of proteolytic activity of bromelain fruit and bromelain stem shows that optimal concentration of cysteine is 0.01-0.02 mole/L (in reaction area with a substrate - 0.004-0.008 mol/l). Further increase of concentration causes the considerable reduction of lysis rate. The speed of casein lysis by bromelain fruit and stem in its concentration 0.5%-2% is unchanged. The rate of casein lysis in both cases is proportional to enzyme concentration within 0.05 g/l-0.25 g/l. The rate of casein lysis is time-proportional in the range 5-20 min. The effect of temperature on the activity of both bromelains (fruit and stem) was studied. It was established that optimal temperature is 55-60ºC in both cases. Thus, investigation allows continuing the work in this direction for preparation of corresponding pharmaceutical product.
Giovanna Bergamini
GSK company, Germany
Title: CZ415, a highly selective mTOR inhibitor showing in vivo efficacy in a collagen induced arthritis (CIA) model
Time : 16:35-17:00

Biography:
Giovanna Bergamini has completed her PhD in Virology at the University of Bologna. She was a Post-doctoral Researcher at the European Molecular Biology Laboratory in Heidelberg, Germany. At Cellzome in Heidelberg, she leads Drug Discovery Programs in the Immuno-inflammation Therapeutic area. As a Director of Discovery Biology following acquisition of Cellzome from GSK, she oversees numerous activities on the investigation of drug mode of action across the GSK portfolio. She has published more than 20 papers in high impact journals.
Abstract:
CZ415, a potent ATP-competitive mTOR inhibitor with unprecedented selectivity over other kinases will be presented here. It’s in vivo pharmacokinetic profile will be reported, in addition to a comprehensive characterization of its in vitro activities and ADME data. The suitability of this inhibitor for studying in vivo mTOR biology is shown by the inhibition of target-dependent phosphorylation signaling observed in a mechanistic mouse model following oral administration. The compound reported here is the first ATP-competitive mTOR inhibitor described to show efficacy in a semi-therapeutic collagen induced arthritis (CIA) mouse model
Jana Sopkova-de Oliveira Santos
University of Caen Normandy, France
Title: ß-Strand Mimicry: Exploring oligothienylpyridine foldamers
Time : 17:00-17:25

Biography:
Jana Sopkova-de Oliveira Santos has completed her PhD from the University Paris XI (Orsay) and University of Charles (Prague). Since 2012, she is a Professor of General Chemistry and Biophysics at the University of Caen Normandy. She has published more than 110 papers in reputed journals
Abstract:
Protein-protein interactions (PPIs) are involved in many cellular processes; consequently, the discovery of small molecules as modulators of PPIs has become a challenge in medicinal chemistry. Structural mimetics of protein secondary structures could maintain or restore biological functions and should possess biological activity. Actually, the most challenging classes of PPIs are those mediated by β-sheet, which are implicated in a number of diseases. Only a few β-strand mimics have been published to date. This study presents an evaluation of oligothienylpyridyl scaffolds in view of their ability for β-strand mimicry. We have observed that a coplanar arrangement in thienylpyridyl systems can be obtained in several different ways. The presence of a nitrogen and sulfur atom in the junction vicinity introduces a coplanar arrangement as well as the presence on the nitrogen atom in the non-orthosubstituted systems. The introduction of an ortho substituent in a system with a nitrogen atom in the junction vicinity perturbs the two rings somewhat, but the system can achieve the coplanar arrangement, because the energy barrier is very low. The same behavior was observed in a non-ortho-substituted biaryl with only a sulfur atom in the junction vicinity. The X-ray structures showed that the compounds have a tendency to adopt a nearly coplanar conformation and the positions of methyl substituents coincide well with those of i, i + 2nd or i, i + 4th β-strand side chains. Therefore, the thienylpyridine scaffold opens the way to produce coplanar compounds mimicking β-strand side-chain distributions.
Lela Amiranashvili
Tbilisi State Medical University, Georgia
Title: Bioactive natural products from Symphytum (Boraginaceae)
Time : 17:25-17:50

Biography:
L Amiranashvili has completed her PhD from I Javakhishvili Tbilisi State University. She is a Research Scientist at Tbilisi State Medical University I Kutateladze Institute of Pharmacochemistry, Department of Plant Biopolymers. She has published more than 40 papers in reputed journals and more than 50 presentations at the international conferences. Her fields of professional interests are Bioorganic and Medicinal Chemistry.
Abstract:
Symphytum asperum and S. caucasicum (prickly or rough comfrey) roots and stems have been used externally as a traditional medicinal plant in treating gastrointestinal and respiratory tract diseases in folk medicine. Previous studies showed that these beneficial properties of comfrey are the result of the presence of numerous bioactive compounds. High-molecular fractions from comfrey were isolated. Based on the IR and NMR spectroscopy data, poly[3-(3,4 dihydroxyphenyl)glyceric acid] (PDPGA) was confirmed to be the major component of these fractions. PDPGA–SA and PDPGA–SC exhibit immunomodulatory (anticomplementary), antioxidant and anti-inflammatory activities and wound-healing property. Phytochemical study of roots/stems of Symphytum asperum Lepech., was carried out in order to define other phenolic constituents. The solid-liquid extraction technique was chosen as the first step for isolation the compounds probably containing the fragments of PDPGA followed by the investigation of the composition of the extracts of S. asperum roots/stems using UHPLC-Q-TOF/MS method. Ultrahigh-pressure liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry (UHPLC-Q-TOF/MS) analysis of extracts of S. asperum roots/ stems was carried out and revealed the presence of low molecular weight compounds such as caffeic, rosmarinic, chlorogenic and salvianolic acids and several oligomeric compounds. The obtained results showed that the comfrey roots/stems can be used as a source for the isolation of low molecular weight biologically active compounds.
Laleh Alisaraie
Memorial University, Canada
Title: In Vitro and In Silico Studies of Peptide Inhibitors of Antibiotic Resistance Enzymes
Time : 17:50-18:15

Biography:
Laleh Alisaraie is an Assistant Professor with the School of Pharmacy, Memorial University of Newfoundland, St. John’s, Canada.
Abstract:
Antibiotic drugs have had an extensive use in recent medical history for their ability to fight infectious diseases. Aminoglycoside antibiotics target gram positive and negative bacteria and are beneficial for several varying medical diseases. However, misuse or overuse of these drugs has resulted in creation and over expression of aminoglycoside modifying enzymes (AMEs), which alter the drugs chemical structure, preventing the antibiotic from reaching the host RNA. The primary focus of this research is the mechanism of chemical alteration of the aminoglycoside antibiotic paromomycin-I by the 3',5" phosphotransferase-IIIa AME and the inhibitory effect of a group of antimicrobial peptides on this enzyme. Molecular docking and Molecular Dynamics (MD) simulations were employed to study the binding mode of selected antibiotics and antibacterial peptides against the phosphotransferase-IIIa enzyme. The analyses of both the conformational changes of the antibiotics ternary complexes and the peptide with the best binding affinity to the enzyme in the presence of ATP were performed usin GROMOS 53a6 force field parameters with SPC water model. An antibacterial peptide, chosen among a small library of selected peptides, yielded a greater binding affinity to the antibiotic binding site than the chosen antibiotics. As well, this peptide contained residues that were determined to occupy the similar space as the antibiotic, yielding an inhibition capability. The different and unique confirmations of the AME were also determined when bound with the either the antibiotic or peptide. The results from this research provide a novel insight into the structure of the enzyme and was able to address questions that were not possible to answer based solely on the available data from the enzymes solved crystal structure. There is currently little information regarding these complexes and the potential antibacterial peptide inhibitors. These analyses will help bind the gap between the available data for future pharmaceutical research and the overall goal of antibiotic resistance prevention.
- Medicinal Chemistry|Chemical Biology|Computer Aided Drug Design

Chair
Gaia Cecchi
University of Florence, Italy

Co-Chair
Monika I Konaklieva
American University, USA
Session Introduction
Concepción González-Bello
Universidade de Santiago de Compostela, Spain
Title: Disabling essential enzyme motion for catalysis: An efficient approach for shikimate kinase inhibition
Time : 15:50-16:15

Biography:
Concepción González-Bello has obtained her PhD from the University of Santiago de Compostela (USC, Spain) in 1994. She did two Pre-doctoral stays at the University of Gent (Belgium) with professor vandewalle and at the Scripps Research Institute (USA) with professor Nicolaou. After a Post-doctoral stay at the University of Cambridge (UK) with professor Abell, she joined USC as an assistant professor, was promoted to associate professor in 2003 and obtained the Spanish habilitation to Full professor in 2011. She joined the Center for Research in Biological Chemistry and Molecular Materials (CIQUS) as a group leader in 2011. She is the author of about 80 papers and several book chapters and an Academic Editor of Plos One. Her main research interest is to develop updated therapies targeting infectious diseases, in particular, drugs with new mechanisms of action to combat the growth of antibiotic-resistant bacteria.
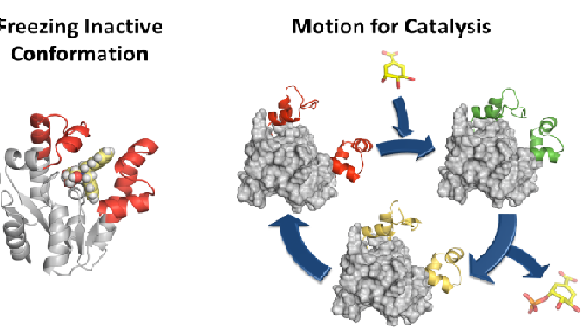
Abstract:
Although antibiotics are one of the most successful drugs in clinic that have saved millions of lives, many of them are nowadays ineffective in treating infections caused by resistant bacteria. It is therefore urgent to search for new antibacterial agents and approaches to face this huge challenge. Considering that most current antibiotics that are highly successful in human clinical use, targeted at only four main key processes and resistance to these antibiotics is widespread and well known, the search for unexplored bacterial functions appears to be a good option for the development of novel antimicrobial agents with a new mechanism of action. Our group is studying the possible development of new antibiotics by the selective and effective inhibition of an essential enzyme in bacteria that does not have any counterpart in human cells, shikimate kinase (SK, aroK gene). In particular, we are focused on SK from M. tuberculosis and H. pylori, two important pathogenic bacteria. Based on the essential enzyme motion for catalysis and product release studied by molecular dynamics simulation studies, potent reversible competitive inhibitors of the enzyme were developed. Compounds that stabilize the closed conformation for catalysis or the open conformation for product release were developed. An ester prodrug approach was used for achieving good in vitro activities against H. pylori. Our results also show that the less exploited motion-based design approach, not only is an alternative strategy for the development of competitive inhibitors, but could also be a way to achieve selectivity against a particular enzyme among its homologous ones. By using this approach, (1) the presence in the selected pocket of residues with markedly different properties would not be required, and (2) the effects of changes on residues to avoid the inhibition (resistance) should have a less pronounced effect.
Recent Publications
1.Blanco B, Prado V, Lence E, Otero J M, Garcia-Doval C, van Raaij M J, Llamas-Saiz A L, Lamb H, Hawkins A R, González-Bello C (2013) Mycobacterium tuberculosis shikimate kinase inhibitors: Design and simulation studies of the catalytic turnover. J. Am. Chem. Soc. 135: 12366-12376.
2.Prado V, Lence E, Maneiro M, Vázquez-Ucha J C, Beceiro A, Thompson P, Hawkins A R, González-Bello C (2016) Targeting the motion of shikimate kinase: Development of competitive inhibitors that stabilize an inactive open conformation of the enzyme. J. Med. Chem. 59: 5471-5487.
3.Prado V, Lence E, Thompson P, Hawkins A R, González-Bello C (2016) Freezing the dynamic gap for selectivity - motion-based design of inhibitors of the shikimate kinase enzyme. Chem. Eur. J. 22: 17988-18000.
4.Prado V, Lence E, Vallejo J A, Beceiro A, Thompson P, Hawkins A R, González-Bello C (2016) Study of the Phosphoryl-transfer mechanism of shikimate kinase by NMR spectroscopy. Chem. Eur. J. 22: 2758-2768.
5.González-Bello C (2016) Inhibition of shikimate kinase and type II dehydroquinase for antibiotic discovery: Structure-based design and simulation studies. Curr. Top Med. Chem. 16: 960-977.
Fabiola Porta
University of Basel, Switzerland
Title: Enzyme triggered nanomaterial for cancer therapy
Time : 16:15-16-40

Biography:
Fabiola Porta has studied Medicinal Chemistry and Pharmaceutical Technology in Milan from the Universita’ degli studi, where she obtained her Master’s degree in Pharmacy in the year 2008. She then moved to the Leiden Institute of Chemistry, where she graduated in Chemistry with a special focus on nanoparticles synthesis and characterization in the year 2012. She has done her post-doctoral studies from FHNW and University of Basel. Recently, she joined the group of Bio-pharmacy, where she is developing-self assembled nanoparticles for cancer therapy. She has expertise in the design and development of nano-vehicles for cancer therapy with a particular focus on targeted nanocarriers for breast cancer therapy.
Abstract:
Polymer vesicles are attracting much attention as alternative nano-delivery system to implement drug targeting strategies. Polymersomes have several interesting features. For instance, ease of chemical modification of the polymer chains can be used to modulate their tissue specificity and organ distribution. A wide variety of polymers is available, however a good candidate for pharmaceutical formulations is the di-block copolymer poly(dimethylsiloxane)-b-poly(2methyloxazoline) (PDMS-PMOXA). This polymer is formed by two subunits which are FDA approved for the development of novel nanomaterials with a potential human use. In our work, we are developing a responsive nanomaterial for the treatment of breast cancer. In order to develop a targeted
delivery system a very good understanding of the cancer biology is necessary. In particular cancer biomarker are of particular interest due to their specificity for cancer cells. The enzyme family of cathepsins is highly expressed in certain type of cancers as breast tumor. Moreover, they are responsible for the degradation of proteins in the lysosomes. The elevate expression of these enzymes in tumors is an evidence of the increased metabolism of cancer cells. For this reason, these enzymes are a very interesting target for the development of a novel nanomaterial. In this work, we are presenting a peptide cross linked polymeric nanoparticle with the main goal to encapsulate anticancer compounds and to release them only upon activation of the system by cathepsin B.
Recent Publications
1.Blanco B, Prado V, Lence E, Otero J M, Garcia-Doval C, van Raaij M J, Llamas-Saiz A L, Lamb H, Hawkins A R, González-Bello C (2013) Mycobacterium tuberculosis shikimate kinase inhibitors: Design and simulation studies of the catalytic turnover. J. Am. Chem. Soc. 135: 12366-12376.
2.Prado V, Lence E, Maneiro M, Vázquez-Ucha J C, Beceiro A, Thompson P, Hawkins A R, González-Bello C (2016) Targeting the motion of shikimate kinase: Development of competitive inhibitors that stabilize an inactive open conformation of the enzyme. J. Med. Chem. 59: 5471-5487.
3.Prado V, Lence E, Thompson P, Hawkins A R, González-Bello C (2016) Freezing the dynamic gap for selectivity - motion-based design of inhibitors of the shikimate kinase enzyme. Chem. Eur. J. 22: 17988-18000.
4.Prado V, Lence E, Vallejo J A, Beceiro A, Thompson P, Hawkins A R, González-Bello C (2016) Study of the Phosphoryl-transfer mechanism of shikimate kinase by NMR spectroscopy. Chem. Eur. J. 22: 2758-2768.
5.González-Bello C (2016) Inhibition of shikimate kinase and type II dehydroquinase for antibiotic discovery: Structure-based design and simulation studies. Curr. Top Med. Chem. 16: 960-977.
Monika I Konaklieva
American University, USA
Title: A new class of b-lactam antibiotics active against drug-sensitive and drug-resistant Mycobacterium tuberculosis
Time : 16:40-17:05

Biography:
Monika I Konaklieva has completed her PhD in Chemistry from SUNY Buffalo in 1997, and became a visiting professor in Medicinal Chemistry at Midwestern University, Chicago, Illinois (1997-1999). She is currently an associate professor at the American University. She has published more than 40 papers in reputed journals and has been serving as an Editorial Board Member of several chemistry journals publishing in the areas of organic and medicinal chemistry.
Abstract:
We have designed, synthesized, and tested monocyclic β-lactams that carry aryl-thioether group at C4. These lactams have shown good intrinsic activity against serine β-lactamase producing Mycobacterium tuberculosis H37Rv (Mtb). Some of the compounds have demonstrated minimal inhibitory concentration (MIC) as low as 6.25 μg/ml in 7H9 and 0.19 μg/ml in GAST. Our investigations indicate that these compounds are cidal to both replicating and non-replicating persistent Mtb. These compounds have also shown activity against multi-drug resistant strains of M. tuberculosis. Therefore, they are promising candidates for lead discovery. Mechanism of action and target identification studies are currently underway.
Michele Tonelli
University of Genoa, Italy
Title: Host DHFR-directed cycloguanil analogues endowed with promising activity against influenza virus and respiratory syncytial virus
Time : 17:05-17:30

Biography:
Michele Tonelli has done his graduation in chemistry and pharmaceutical technology from Genoa University in the year 2003. He did his PhD in pharmaceutical food and cosmetic sciences. He has participated in many national research projects. Since 2012, he is an Assistant Professor (Medicinal Chemistry) in the Department of Pharmacy, University of Genoa. He has published more than 25 papers in reputed journals and has been serving as Reviewer of Journal of Medicinal Chemistry, Bioorganic & Medicinal Chemistry, Antiviral Research, Future Medicinal Chemistry, etc.
Abstract:
The Orthomyxoviridae and Paramyxoviridae families comprise important respiratory pathogens, such as respiratory syncytial virus, influenza A and B viruses. The acute respiratory illnesses caused by these viruses represent a major medical need. In particular, the risk for a severe influenza A pandemic leads to the development of new and broadly acting therapeutics an urgent issue. Currently, used antiviral drugs preferentially inhibit virus-specific replication factors. An alternative and emerging strategy is to address host factors involved in virus replication, because less susceptible to mutate. Herein, we have identified a series of 4,6-diamino- 1,2-dihydrotriazines, structurally related to the antimalarial drug cycloguanil, as new inhibitors of influenza A and B virus and RSV via targeting of the host dihydrofolate reductase (DHFR) enzyme. They proved active against influenza B virus in the low micromolar
range, sometimes reaching the sub-micromolar potency of zanamivir (EC50=0.060 μM), and markedly exceeded (up to 327 times) the antiviral efficacy of ribavirin. Besides inhibiting two influenza A strains, more importantly the compounds displayed nanomolar activity against RSV with a SI (CC50/EC50)>10,000 for the most active compounds (EC50~0.008 μM), far surpassing the potency and safety profile of the licensed drug ribavirin (EC50=5.8 μM, SI>43). These compounds, tested against the recombinant protein of the hDHFR, also confirmed to bind this enzyme in the sub-micromolar range. Kinetic inhibition studies showed a competitive inhibition behavior, and docking studies disclosed the most probable binding mode for this class of compounds as hDHFR ligands.
